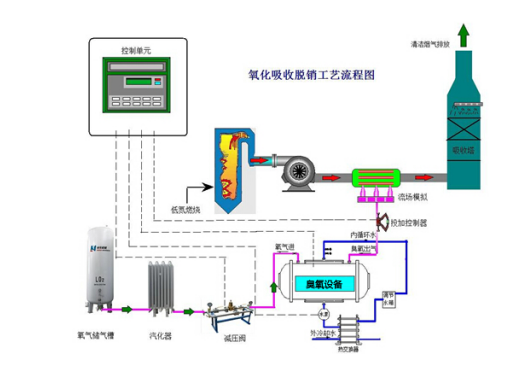
Ozone, as a strong oxidant, can easily oxidize NO, which is hard to dissolve in water, into high valence nitrogen oxides such as NO2, N2O3 and N2O5, which can be dissolved in water. Then, Na2S and NaOH were used in the washing tower to absorb. Finally, NOX was converted to N2 to achieve the purpose of removal, and the removal rate of NOX was as high as 95%. The influencing factors of ozone denitration include mole ratio, reaction temperature, reaction time, the nature of absorption liquid, and ozone dosing system.

The ozone gas distribution system is designed in combination with the above factors. In the process of design, ComputationalFluidDynamics computer simulation analysis and optimization are improved, and the original gas pipeline is upgraded to ozone multi point countercurrent jet flue gas distribution system.

The ozone low temperature denitrification system is applied to sintering machine, chain grate furnace, circulating fluidized bed boiler, glass furnace, garbage incinerator and biomass boiler.

Principle of ozone denitrification
According to O3's complex oxidation process for NOx, in fact, it is finally manifested through the change of valence state of N. The main reactions are as follows:
2NO+3O3=N2O5+3O2
2N02+O3=N2O5+O2
NO+O3=NO2+O2
Compared with other chemical substances in the gas phase, such as CO and SOx, NOx can be oxidized rapidly by ozone, which makes NOx ozonation highly selective. Because the NOx in the gas phase is converted into an ionic compound dissolved in the aqueous solution, this makes the oxidation reaction more complete, thereby irreversibly removing the NOx, without two pollution.
After the oxidation reaction, the ozone added is consumed by the reaction, and the excessive ozone can be decomposed in the spray tower. In addition to NOx, some heavy metals, such as mercury and other heavy metal pollutants, are also oxidized by ozone. High concentration dust or solid particles in flue gas will not affect the removal efficiency of NOx.

Process classification
The ozone generator is a high voltage discharge generator, which uses high voltage current of a certain frequency to produce high voltage corona electric field, which makes the oxygen molecules in the electric field or around the electric field to have electrochemical reaction, thus making ozone.
VPSA air separation: the compressed air is used as the gas source. After filtration, it enters the adsorption tower with zeolite molecular sieve and produces high purity oxygen into the ozone generator.
Liquid oxygen source: liquid oxygen is used as the source of air, and after decompression and gasification, oxygen is generated into the ozone generator.
A schematic diagram of the process of ozonation and denitrification